Dr. Megan Turner is a third-year general surgery resident at Duke University in Durham, North Carolina. She went to medical school at the University of Washington in Seattle. Dr. Turner’s academic interests include perioperative decision making, behavioral economics, gender in the workplace, and outcomes research.

Dr. Christopher Mantyh is the Chief of Colorectal Surgery and Professor of Surgery at Duke University Medical Center. He has recently been named as the Chief of Quality for the Department of Surgery. Dr. Mantyh has implemented changes to improve best practices in surgical procedures performed at Duke Hospital using multiple platforms including the National Surgical Quality Improvement Project, for which he is the Surgical Champion. In this role, he has championed a quality improvement plan based on the surgical bundles and early recovery after surgery protocol that has led to significant improvements in clinical patient outcomes and cost savings. In addition, his research over the last five years has been focused on quality indexes and outcomes in colorectal cancer. Dr. Mantyh’s multidisciplinary work has spanned the spectrum of treatment of colorectal cancer from novel endoscopic imaging of colonic dysplasia, to proper operative technique, and evaluation of molecular markers of colorectal cancer to help determine individualized chemotherapy. Collaborative efforts include working closely with bioengineering, radiation oncology, medical oncology, statisticians, and physiologists. Recently, his group has investigated large national databases to develop predictive modeling systems to anticipate surgical complications. Dr. Mantyh is currently funded through multiple foundation and national grants. He has written over 130 peer-reviewed publications and book chapters.
Turner and Mantyh_Current Dialogues in Wound Management_2018_Volume 4_Issue 4
INTRODUCTION
A pressure injury (PI) is a wound over a bony prominence that occurs in the setting of immobility, altered consciousness, and illness with an underlying pathophysiologic pathway of ischemia.1-3 These wounds are commonly encountered in a general surgery practice and are becoming a measure of hospital quality by which reimbursements and performance ranking are based.4-6 While definitive management of the defect is surgical flap coverage, many of these wounds are not appropriate for this intervention, and high rates of recurrence persist.7 While challenging, persistent attention to treatment is imperative as these do not spontaneously regress, and frequently progress to sepsis. This paper will address staging of PIs, contraindications for flap coverage, and surgical management principles for stage III and IV ulcerations.
DEFINITION
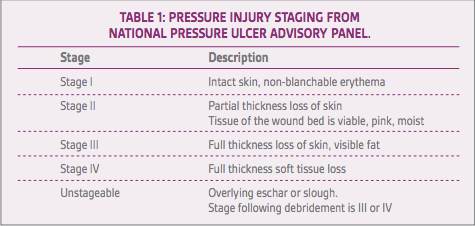
PIs have specific criteria for staging as described by the National Pressure Ulcer Advisory Panel.8 The pathophysiology includes compromised arterial inflow, venous outflow, and shear forces along the tissue planes over a fixed bony prominence. Stage I includes wounds with intact skin with non-blanchable erythema. Stage II includes partial thickness loss of skin, where the tissue of the wound bed is viable, pink, and moist. Stage III wounds exhibit full thickness loss of skin with visible fat. Slough or eschar may be visible, as well as undermining or tunneling of the surrounding tissue. Stage IV wounds are full thickness skin and tissue loss and can be down to the level of the muscle, bone, tendon, or fascia. Unstageable PIs include those where the extent of the tissue loss cannot be determined secondary to overlying eschar or slough. Following debridement, these injuries are staged as III and IV (Table 1). Deep PIs appear as localized areas of persistent non-blanchable discoloration or epidermal separation withblistering. Further resources on staging PIs can be found at the National Pressure Ulcer Advisory Panel website.
SURGICAL CONSULTATION
Patients with injuries that are stage III and IV should be evaluated by surgical teams for determining next steps of therapy. Patients who exhibit the following characteristics should be optimized prior to attempting flap coverage of the defect, or use one of the described strategies as destination therapy if they are deemed too ill for definitive management:
•Patients with persistent infection, not colonization, of the wound bed, including osteomyelitis of the underlying bone.
•Patients who have sustained pressure related to immobility.
•Patients with poor management of stool and incontinence.
•Patients with malnutrition.
•Patients with lack of access to wound care.
Patients who smoke tobacco are ineligible for flap coverage as tobacco abuse is associated with flap failure and recurrence of wounds. Resources should be made available for smoking cessation programs as smoking cessation is beneficial for all patients with chronic wounds.
Patients can be optimized and managed, as described in the remainder of this article, to prevent infection, improve quality of life, and increase the chance they will qualify for definitive management.9 Current practice patterns have high variability but should follow the same principles.
PRESSURE DISTRIBUTION
PI prevention and healing optimization can occur when adequate pressure distribution over firm points is obtained. There is inconclusive evidence for the use of powered over non-powered pressure distribution bedding.10,11Current standard of care includes management of pressure as well as moisture and shear forces which can be mitigated using a turning schedule.
INFECTION MANAGEMENT
Infection management begins with source control. Hemodynamic instability from sepsis should prompt urgent/emergent operative debridement of infected, purulent, and necrotic tissue. For patients who are hemodynamically stable, routine debridement can be offered. Osteomyelitis should be debrided as tolerated by the patient recognizing that progression to sepsis dramatically increases mortality.12 Serial trips to the operating room may be required to optimally define healthy tissue. Broad-spectrum antibiotics should be initiated including gram-positive, gram-negative, and anaerobic coverage during critical illness as many of these infections are polymicrobial. Prompt and appropriate narrowing of antibiotics based on culture data or local microbiome should be implemented with the assistance of infectious disease specialists.
Following debridement, local wound care is essential. This may be performed with wet-to-dry dressing changes twice daily, alginate dressings, or foam dressings which are traditionally considered standard of care. Antiseptics may be incorporated into wound dressings at the discretion of the caretaker. Level one data for specific types of dressings for PIs has been challenging to obtain.10,13 Attention to areas of undermining and tunneling is essential to promote healing. Wound management can also be performed with negative pressure wound therapy. This strategy reduces the frequency of dressing changes that may be painful, manages the moisture of the wound, helps remove infectious material, and promotes contraction of the wound size.14-16 The mechanism of action includes increased perfusion, stimulation of granulation tissue, reduction in edema, and decreased bacterial colonization.17 This therapy may be particularly helpful in wounds that are moist or subject to contamination by feces. However, there is a lack of level one evidence to strongly support this practice.
DIVERSION OF FECAL STREAM
Many PIs can become contaminated with stool secondary to incontinence, frailty, and immobility of affected patients. For patients who have recurrent or persistent infections, diversion of the fecal stream may be appropriate for optimal management of the PI and during healing of flap coverage. Recurrence rates as well as time to healing of the wound are decreased.19 End colostomy or loop ileostomy can be performed with limited morbidity in these patients. A laparoscopic or single incision approach further decreases the morbidity of the intervention while successfully diverting the fecal stream. For patients anticipating permanent fecal diversion, end colostomy is preferred with superior pouching and decreased risk for prolapse. Patients anticipating reversal following the resolution of their PI or successful healing following flap coverage may elect for a loop ileostomy. Loop colostomies and end ileostomies can also be successfully performed but are the exception.19Many of our patients find stoma care superior stool management to incontinence or dependence on a caretaker, and permanent diversion should be considered in this group.
NUTRITION
PIs are associated with a chronic disease state. These wounds arise in the setting of overall catabolic state and protein calorie malnutrition. Monitoring nutritional state includes identifying acute weight loss and physical exam findings, such as temporal wasting and scaphoid abdomen. Laboratory studies to measure protein nutrition include serum prealbumin as a short-term measure (half-life 7 days), and albumin and iron studies as longer-term measures (half-life 21 days). Wound size has been shown to decrease in stage III/IV wounds for those receiving appropriate enteral feeds.9,20 Patients will not be eligible for definitive management of their wounds until their nutrition is optimized. This can be facilitated through nutritional consultation and th emonitoring of protein intake with supplementation either orally or with enteral feeding regimens.11 However, targeted supplementation of specific vitamins has inconclusive evidence for expediting wound healing or preventing recurrence.10 With regards to optimizing nutrition for patients with chronic wounds, parenteral nutrition may not be an appropriate strategy. Parenteral nutrition places the patient at risk for infectious complications both at access sites and from their wounds.
CONCLUSIONS
Attention to PI is imperative as it is a measure of hospital quality supported by the Agency for Health Care Research and Quality and Centers for Medicare and Medicaid Services.22 While prevention is the ultimate goal, patients with established PIs not amenable to flap coverage are common surgical consults. The above principles of smoking cessation, pressure distribution, infection management, diversion of fecal stream, and nutritional optimization can improve outcomes for these patients in wound healing and overall quality of life. Provider adherence to the recommended guidelines and patient compliance to patient-controlled factors can increase the likelihood of qualifying for definitive flap coverage and minimize sequelae of chronic disease in these patients.
References
1.Berlowitz DR, Wilking SV. Risk factors for pressure sores. A comparison of cross-sectional and cohort-derived data. J Am Geriatr Soc. 1989;37(11):1043-1050.
2.Rondinelli J, Zuniga S, Kipnis P, Kawar LN, Liu V, Escobar GJ. Hospital-acquired pressure injury: risk-adjusted comparisons in an integrated healthcare delivery system. Nurs Res. 2018;67(1):16-25. doi:10.1097/NNR.0000000000000258.
3.Allman RM. Pressure ulcers among the elderly. N Engl J Med. 1989;320(13):850-853.
4.McNair PD, Luft HS, Bindman AB. Medicare’s policy not to pay for treating hospital-acquired conditions: the impact. Health Aff (Millwood). 2009;28(5):1485-1493.
5.Centers for Medicare and Medicaid Services. Medicaid program; payment adjustment for provider-preventable conditions including health care-acquired conditions. Final rule. Fed Regist. 2011;76(108):32816-32838.
6.Centers for Medicare and Medicaid Services. Hospital-Aquired Conditions. www cms gov. 2018; https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalAcqCond/Hospital-Acquired_Conditions.html. Updated August 30, 2018. Accessed October 24, 2018.
7.Lyder CH. Pressure ulcer prevention and management. JAMA.2003;289(2):223-226.
8.National Pressure Ulcer Advisory Panel. NPUAP Pressure Injury Stages. National Pressure Ulcer Advisory Panel. 2018; http://www.npuap.org/resources/educational-and-clinical-resources/npuap-pressure-injury-stages/. Accessed October 18, 2018.
9.Bergstrom N, Horn SD, Smout RJ, et al. The National Pressure Ulcer Long-Term Care Study: outcomes of pressure ulcer treatments in long-term care. J Am Geriatr Soc. 2005;53(10):1721-1729.
10.Reddy M, Gill SS, Kalkar SR, Wu W, Anderson PJ, Rochon PA. Treatment of pressure ulcers: a systematic review. JAMA. 2008;300(22):2647-2662. doi: 10.1001/jama.2008.778.
11.Smith ME, Totten A, Hickam DH, et al. Pressure ulcer treatment strategies: a systematic comparative effectiveness review. Ann Intern Med. 2013;159(1):39-50. doi:10.7326/0003-4819-159-1-201307020-00007.
12.Andrianasolo J, Ferry T, Boucher F, et al. Pressure ulcer-related pelvic osteomyelitis: evaluation of a two-stage surgical strategy (debridement, negative pressure therapy and flap coverage) with prolonged antimicrobial therapy. BMC Infect Dis. 2018;18(1):166. doi:10.1186/s12879-018-3076-y.
13.Ashby RL, Dumville JC, Soares MO et al. A pilot randomised controlled trial of negative pressure wound therapy to treat grade III/IV pressure ulcers [ISRCTN69032034]. Trials.2012;13(1):119.
14.Moues CM, Van Den Bemd GJ, Heule F, Hovius SE. Comparing conventional gauze therapy to vacuum-assisted closure wound therapy: a prospective randomised trial. J Plast Reconstr Aesthet Surg. 2007;60(6):672-681.
15.de Laat EH, van den Boogaard M, Spauwen PH, van Kuppevelt DH, van Goor H, Schoonhoven L. Faster wound healing with topical negative pressure therapy in difficult-to-heal wounds: A prospective randomized controlled trial. Ann Plast Surg. 2011;67(6):626-631. doi: 10.1097/SAP.0b013e31820b3ac1.
16.Baharestani MM, Houliston-Otto DB, Barnes S. Early versus late initiation of negative pressure wound therapy: examining the impact on home care length of stay. Ostomy Wound Manage. 2008;54(11):48-53.
17.Bovill E, Banwell PE, Teot L et al. Topical negative pressure wound therapy: a review of its role and guidelines for its use in the management of acute wounds. Int Wound J. 2008;5(4):511-529. doi: 10.1111/j.1742-481X.2008.00437.x.
18.Gregor S, Maegele M, Sauerland S, Krahn JF, Peinemann F, Lange S. Negative pressure wound therapy: a vacuum of evidence? Arch Surg 2008;143(2):189-196. doi: 10.1001/archsurg.2007.54.
19.de la Fuente SG, Levin LS, Reynolds JD et al. Elective stoma construction improves outcomes in medically intractable pressure ulcers. Dis Colon Rectum. 2003;46(11):1525-1530. doi:10.1097/01.DCR.0000093641.57817.12.
20.Breslow RA, Hallfrisch J, Guy DG, Crawley B, Goldberg AP. The importance of dietary protein in healing pressure ulcers. J Am Geriatr Soc. 1993;41(4):357-362.
21.Worthington PH, Gilbert KA. Parenteral nutrition: risks, complications, and management. J Infus Nurs. 2012;35(1):52-64. doi:10.1097/NAN.0b013e31823b98ef.
22.Lyder CH, Ayello EA. Pressure Ulcers: A Patient Safety Issue. In: Hughes RG, ed. Patient Safety and Quality: an Evidence-Based Handbook for Nurses. Rockville, MD: Agency for Healthcare Research and Quality; 2008:1-33.